In today's technology-driven world, lithium-ion batteries are almost everywhere, from smartphones to new energy vehicles to large-scale energy storage systems. As the core component of lithium-ion batteries, electrolyte plays the role of "lifeline". This article will deeply analyze the definition, composition and basic requirements of electrolyte to help you understand its key role in lithium-ion batteries.
1. Basic definition of electrolyte
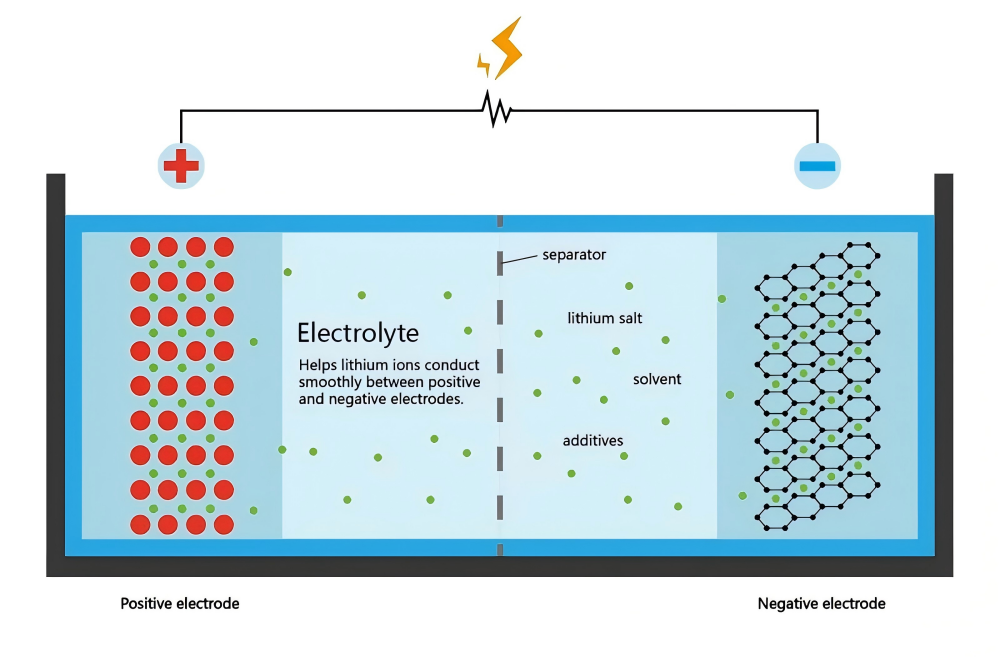
The role of electrolyte in lithium-ion batteries can be likened to a "transportation hub" designed specifically for lithium-ion delivery. Its core definition is a conductive medium mixed with organic solvents, lithium salts and electrolyte additives, designed to maintain the internal ion migration of the battery. Specifically, the electrolyte of lithium-ion batteries must remain stable under various conditions. For example, in lithium iron phosphate batteries such as lifepo4 lithium battery, it provides high safety and long-lasting performance. The emergence of lithium-ion batteries has made electrolytes the focus of research and development, and manufacturers such as well-known lithium ion battery manufacturers are continuously developing more optimized formulas to suit various battery pack integrated systems.
2. Basic composition of electrolyte
The electrolyte of a lithium-ion battery is not a single substance, but a combination of three key components, each of which performs its own function to optimize battery performance. These components include:
(1) Solvent: As a "carrier", the solvent dissolves the lithium salt and provides a migration path for lithium ions. Commonly used solvents such as ethylene carbonate (EC) ensure high conductivity, and combined with dimethyl carbonate (DMC) can improve the charge and discharge efficiency of lithium-ion batteries. In low-temperature environments, the combination of mixed solvents (such as adding DEC) supports the stable operation of the battery pack under harsh conditions.
(2) Lithium Salt: Lithium salt is the source of lithium ions and directly affects the conductivity of the electrolyte. The mainstream LiPF₆ is widely used in lithium-ion batteries and provides good all-round performance. However, emerging lithium salts such as LiFSI are being explored by lithium ion battery manufacturers to enhance thermal stability and safety for advanced systems such as lifepo4 lithium battery.
(3) Additives: These trace ingredients are like "catalysts". Although the amount used is less than 5%, they can greatly improve the performance of the electrolyte. For example, FEC additives can form a stable SEI film in lithium-ion batteries and extend the cycle life; flame retardant additives reduce the safety risks of applications such as battery energy storage systems. Lithium-ion battery manufacturers are using these additives to break through the bottlenecks of high temperature and high voltage.
(1) Solvent: As a "carrier", the solvent dissolves the lithium salt and provides a migration path for lithium ions. Commonly used solvents such as ethylene carbonate (EC) ensure high conductivity, and combined with dimethyl carbonate (DMC) can improve the charge and discharge efficiency of lithium-ion batteries. In low-temperature environments, the combination of mixed solvents (such as adding DEC) supports the stable operation of the battery pack under harsh conditions.
(2) Lithium Salt: Lithium salt is the source of lithium ions and directly affects the conductivity of the electrolyte. The mainstream LiPF₆ is widely used in lithium-ion batteries and provides good all-round performance. However, emerging lithium salts such as LiFSI are being explored by lithium ion battery manufacturers to enhance thermal stability and safety for advanced systems such as lifepo4 lithium battery.
(3) Additives: These trace ingredients are like "catalysts". Although the amount used is less than 5%, they can greatly improve the performance of the electrolyte. For example, FEC additives can form a stable SEI film in lithium-ion batteries and extend the cycle life; flame retardant additives reduce the safety risks of applications such as battery energy storage systems. Lithium-ion battery manufacturers are using these additives to break through the bottlenecks of high temperature and high voltage.
3. Basic performance requirements of electrolyte
According to industry standards, a qualified electrolyte must meet high requirements in multiple dimensions, as emphasized in the core design of lithium-ion batteries. These requirements are rooted in the reliability, safety and efficiency of the battery. Based on key performance dimensions, the basic requirements of electrolytes in lithium-ion batteries include:
(1) Conductivity: Achieve high ionic conductivity, which is to speed up the charging and discharging process. Without it, the efficiency of lithium-ion batteries will drop significantly, affecting the performance of the overall battery pack.
(2) Chemical stability: The electrolyte must coexist stably with positive and negative electrode materials (such as graphite or nickel cobalt) without harmful side reactions. This requirement is particularly prominent in the lifepo4 lithium battery because of its strong chemical tolerance.
(3) Thermal stability: Remaining stable and non-decomposing at high temperatures is crucial to ensuring the safety of battery energy storage systems. This prevents the risk of fire when the lithium-ion battery is under high load.
(4) Electrochemical stability: Supports high voltage operation (such as above 4.4V), suitable for high energy density lithium-ion batteries such as ternary lithium, helping to extend the life of the battery pack.
(5) Safety and low temperature performance: The electrolyte must be non-flammable and low-toxic, and maintain conductivity at -20°C. The use of lithium-ion batteries in winter electric vehicles depends on this to ensure that the battery pack can start in severe cold. The emergence of lithium-ion batteries has emphasized the stringency of these requirements, and developers such as lithium ion battery manufacturers are constantly innovating, such as developing more efficient formulas for energy storage systems.
(1) Conductivity: Achieve high ionic conductivity, which is to speed up the charging and discharging process. Without it, the efficiency of lithium-ion batteries will drop significantly, affecting the performance of the overall battery pack.
(2) Chemical stability: The electrolyte must coexist stably with positive and negative electrode materials (such as graphite or nickel cobalt) without harmful side reactions. This requirement is particularly prominent in the lifepo4 lithium battery because of its strong chemical tolerance.
(3) Thermal stability: Remaining stable and non-decomposing at high temperatures is crucial to ensuring the safety of battery energy storage systems. This prevents the risk of fire when the lithium-ion battery is under high load.
(4) Electrochemical stability: Supports high voltage operation (such as above 4.4V), suitable for high energy density lithium-ion batteries such as ternary lithium, helping to extend the life of the battery pack.
(5) Safety and low temperature performance: The electrolyte must be non-flammable and low-toxic, and maintain conductivity at -20°C. The use of lithium-ion batteries in winter electric vehicles depends on this to ensure that the battery pack can start in severe cold. The emergence of lithium-ion batteries has emphasized the stringency of these requirements, and developers such as lithium ion battery manufacturers are constantly innovating, such as developing more efficient formulas for energy storage systems.
4. Importance and Examples of Electrolyte
The role of electrolyte in lithium-ion batteries cannot be underestimated. It directly determines the applicability of the system in various scenarios. Taking the lifepo4 lithium battery as an example, the special composition of the electrolyte supports the safety and long cycle life of lithium iron phosphate, making it a common choice for household battery energy storage systems.
(1) In high-energy density batteries (such as ternary lithium batteries), the electrolyte must adapt to operating voltages above 4.4V, requiring a wider electrochemical window.
(2) In low-temperature applications (such as winter start-up of electric vehicles), the electrolyte is required to have good fluidity and ion mobility at low temperatures.
(3) In cutting-edge technologies such as solid-state batteries and silicon anode batteries, the composition and design of the electrolyte are constantly being broken through and optimized. Lithium ion battery manufacturers are increasing their investment in this regard, with goals including a wider temperature window and higher safety standards.
In short, the electrolyte is obscure, but it is the core pillar of lithium-ion battery performance, safety and life. From daily consumer electronics to large-scale battery energy storage systems, it allows the entire battery pack system to work in harmony. As the new energy revolution accelerates, the demand for lithium-ion batteries has surged (lithium-ion batteries have been mentioned more than 15 times in this article), and innovation in electrolytes will become the key - wider voltage compatibility, enhanced low-temperature performance, and environmentally friendly formulas. For lithium ion battery manufacturers, choosing the right electrolyte is not only about improving battery efficiency, but also a step to embrace a sustainable future.
(1) In high-energy density batteries (such as ternary lithium batteries), the electrolyte must adapt to operating voltages above 4.4V, requiring a wider electrochemical window.
(2) In low-temperature applications (such as winter start-up of electric vehicles), the electrolyte is required to have good fluidity and ion mobility at low temperatures.
(3) In cutting-edge technologies such as solid-state batteries and silicon anode batteries, the composition and design of the electrolyte are constantly being broken through and optimized. Lithium ion battery manufacturers are increasing their investment in this regard, with goals including a wider temperature window and higher safety standards.
In short, the electrolyte is obscure, but it is the core pillar of lithium-ion battery performance, safety and life. From daily consumer electronics to large-scale battery energy storage systems, it allows the entire battery pack system to work in harmony. As the new energy revolution accelerates, the demand for lithium-ion batteries has surged (lithium-ion batteries have been mentioned more than 15 times in this article), and innovation in electrolytes will become the key - wider voltage compatibility, enhanced low-temperature performance, and environmentally friendly formulas. For lithium ion battery manufacturers, choosing the right electrolyte is not only about improving battery efficiency, but also a step to embrace a sustainable future.
 +86 13332949210
+86 13332949210 info@xihobattery.com
info@xihobattery.com







 Xiho
Xiho Jul 14 2025
Jul 14 2025