Uncover the core role of SEI and CEI in lithium batteries (Li-ion Battery) and the unique advantages of lifepo4 batteries in this field.
As the core technology of modern energy storage, lithium-ion batteries are widely used in electric vehicles, energy storage systems, consumer electronics and other fields. However, the performance degradation and safety hazards of lithium batteries have always been the focus of industry attention. Among them, SEI (Solid Electrolyte Interphase) and CEI (Cathode Electrolyte Interphase) are key factors affecting the life and stability of lithium batteries. This article will deeply analyze the role of SEI and CEI, and explore the unique advantages of LiFePO4 batteries in this field.
What is SEI?
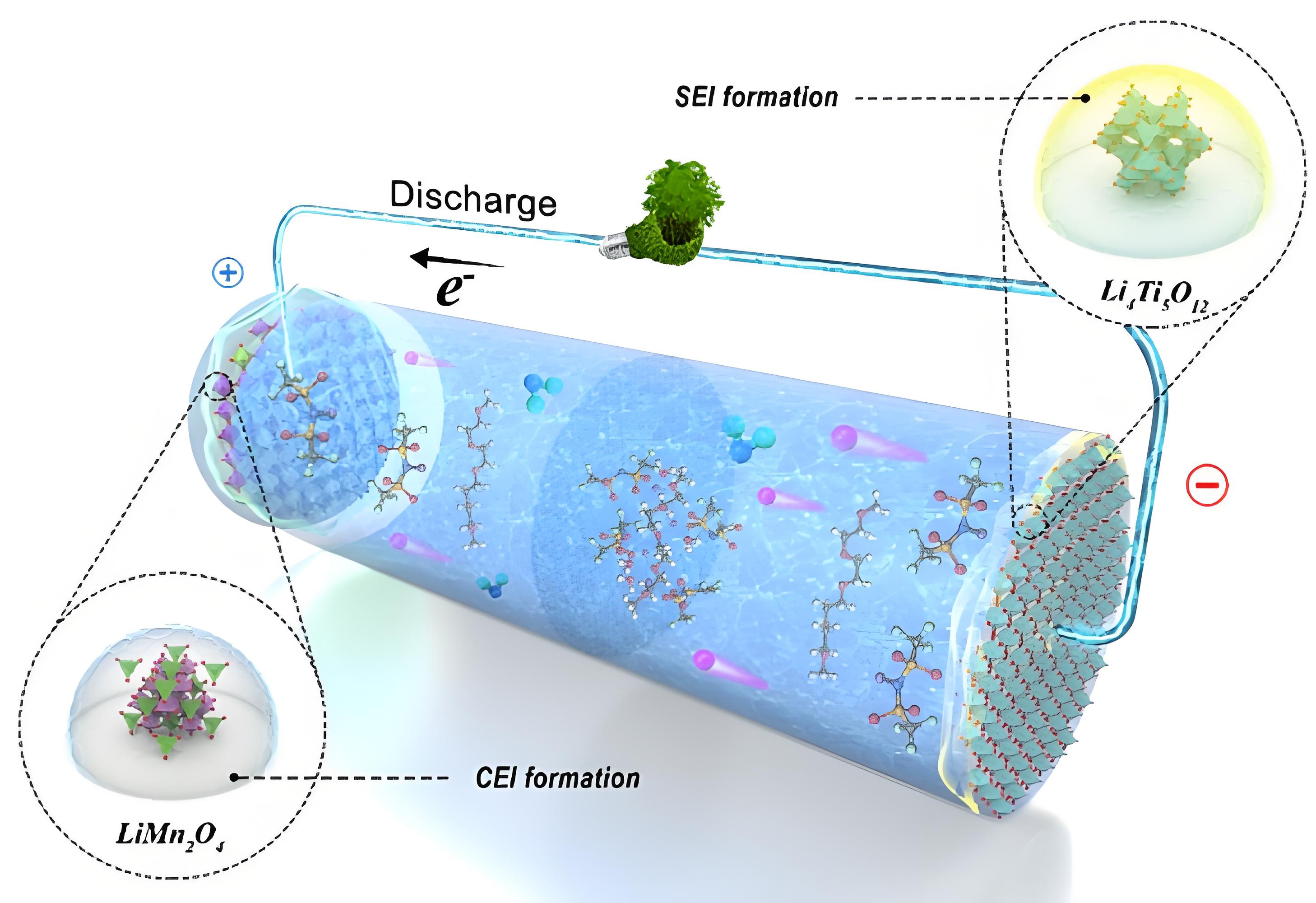
SEI is a solid electrolyte interface film formed on the surface of the negative electrode of a lithium battery. When a lithium battery is charged for the first time, the lithium salt in the electrolyte reacts with the electrode material to form an SEI layer. This film has the characteristics of ion conduction and electronic insulation, which can prevent the electrolyte from continuously decomposing while allowing lithium ions to embed into the negative electrode. The stability of SEI directly determines the cycle life of a lithium battery: if SEI is repeatedly broken or thickened, it will cause lithium dendrites to grow and accelerate capacity decay.
The formation conditions of SEI in lithium batteries are closely related to the electrolyte composition, temperature and negative electrode materials. For example, the SEI of graphite negative electrodes is usually denser, while the SEI of silicon-based negative electrodes is more prone to cracking due to large volume expansion. Therefore, optimizing SEI is one of the core directions for improving the performance of lithium batteries.
The formation conditions of SEI in lithium batteries are closely related to the electrolyte composition, temperature and negative electrode materials. For example, the SEI of graphite negative electrodes is usually denser, while the SEI of silicon-based negative electrodes is more prone to cracking due to large volume expansion. Therefore, optimizing SEI is one of the core directions for improving the performance of lithium batteries.
What is CEI?
CEI (Cathode Electrolyte Interface) is a protective layer on the surface of the positive electrode of a lithium battery. Unlike SEI, the formation of CEI is more due to the oxidation reaction between the electrolyte and the positive electrode material under high voltage. The role of CEI is to prevent the collapse of the positive electrode material structure and the dissolution of transition metals, thereby maintaining the high-voltage stability of the lithium battery. For example, in a ternary lithium battery, the integrity of CEI directly affects the thermal safety and rate performance of the battery.
However, the research on CEI of lithium batteries still faces challenges. Compared with SEI, the composition of CEI is more complex and is easily affected by electrochemical environment fluctuations. Developing artificial CEI coatings or improving electrolyte formulations are the key to breaking through the bottleneck of high-nickel cathode materials in the current industry.
However, the research on CEI of lithium batteries still faces challenges. Compared with SEI, the composition of CEI is more complex and is easily affected by electrochemical environment fluctuations. Developing artificial CEI coatings or improving electrolyte formulations are the key to breaking through the bottleneck of high-nickel cathode materials in the current industry.
LiFePO4 battery's natural advantages in SEI and CEI
Among many lithium battery systems, LiFePO4 battery has relatively low requirements for SEI and CEI due to its unique olivine structure.
(1) Stronger SEI stability: LiFePO4 has a lower lithium insertion potential, a lower risk of electrolyte oxidation, and SEI is less likely to decompose.
(2) Long cycle life: The cycle life of LiFePO4 batteries can reach more than 3,000 times, partly due to its stable SEI and cathode material structure.
(3) High thermal safety: Even if the CEI fails locally, the risk of thermal runaway of LiFePO4 is much lower than that of ternary materials, making it suitable for safety-sensitive scenarios such as energy storage.
SEI and CEI are the "invisible shields" of lithium batteries, guarding the stability of the negative electrode and the positive electrode respectively. Optimizing SEI can improve the cycle efficiency of lithium batteries, while improving CEI can help expand the application boundaries of high-voltage batteries. At the same time, LiFePO4 batteries have become a popular choice in large-scale energy storage and electric vehicles due to their natural SEI/CEI stability.
(1) Stronger SEI stability: LiFePO4 has a lower lithium insertion potential, a lower risk of electrolyte oxidation, and SEI is less likely to decompose.
(2) Long cycle life: The cycle life of LiFePO4 batteries can reach more than 3,000 times, partly due to its stable SEI and cathode material structure.
(3) High thermal safety: Even if the CEI fails locally, the risk of thermal runaway of LiFePO4 is much lower than that of ternary materials, making it suitable for safety-sensitive scenarios such as energy storage.
SEI and CEI are the "invisible shields" of lithium batteries, guarding the stability of the negative electrode and the positive electrode respectively. Optimizing SEI can improve the cycle efficiency of lithium batteries, while improving CEI can help expand the application boundaries of high-voltage batteries. At the same time, LiFePO4 batteries have become a popular choice in large-scale energy storage and electric vehicles due to their natural SEI/CEI stability.
 +86 13332949210
+86 13332949210 info@xihobattery.com
info@xihobattery.com







 Xiho
Xiho May 08 2025
May 08 2025